How Many Atoms Are In Co
tiburonesde
Dec 06, 2025 · 11 min read
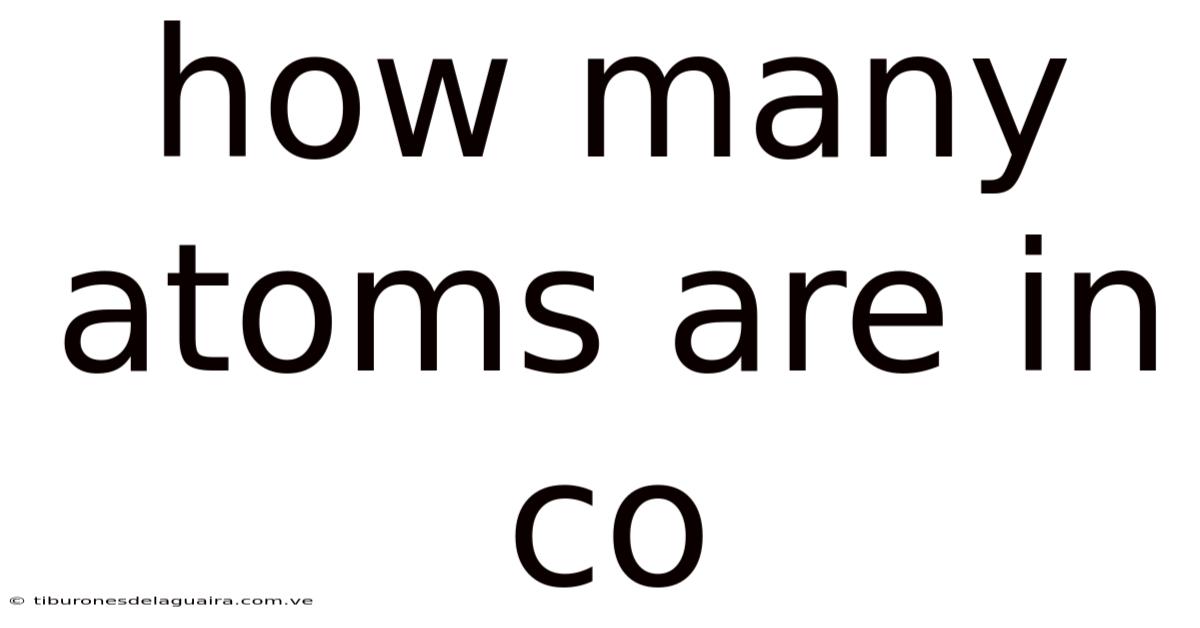
Table of Contents
Imagine holding a single breath on a crisp winter morning, watching the vapor dissipate into the air. Within that seemingly simple exhalation lies a complexity that stretches down to the very building blocks of matter: atoms. Just as a grand building is constructed from individual bricks, all matter, including the carbon monoxide (CO) in that breath, is made up of atoms. Understanding the atomic composition of even the simplest molecules like CO opens a gateway to appreciating the intricate and awe-inspiring structure of the universe.
Have you ever wondered what lies beneath the surface of the air we breathe, the pollutants we fear, or the life-sustaining compounds that keep us alive? At the heart of it all are atoms, the fundamental units that define every element and compound. In the case of carbon monoxide (CO), a seemingly simple molecule, the question of "how many atoms are in CO" leads us to a deeper exploration of molecular composition, atomic structure, and the very essence of chemical compounds. This article aims to demystify the atomic makeup of CO, providing a comprehensive look at its components, significance, and the underlying principles that govern its existence.
Main Subheading
Carbon monoxide (CO) is a colorless, odorless, and tasteless gas produced by the incomplete combustion of carbon-containing fuels, such as gasoline, wood, propane, and natural gas. Often dubbed the "silent killer," CO is notoriously dangerous because it is virtually undetectable by human senses, and it can rapidly accumulate in enclosed spaces, leading to severe health risks and even death. Understanding its chemical structure is crucial not only for appreciating its properties but also for recognizing the dangers it poses.
The simple formula 'CO' tells us a great deal. It reveals that a molecule of carbon monoxide consists of one carbon atom (C) and one oxygen atom (O). This seemingly straightforward combination, however, belies the complex interactions and properties that arise from the bonding of these two atoms. Each carbon and oxygen atom brings its unique characteristics, influencing the overall behavior and impact of the CO molecule. The purpose of this article is to explore these underlying concepts and shed light on the essential aspects of CO’s atomic composition.
Comprehensive Overview
Atomic Structure Basics
Atoms are the basic building blocks of matter, comprising a central nucleus surrounded by electrons. The nucleus contains protons (positively charged particles) and neutrons (neutral particles), while electrons (negatively charged particles) orbit the nucleus in specific energy levels or shells. The number of protons in an atom's nucleus defines the element and is known as the atomic number.
Carbon (C)
Carbon, represented by the symbol 'C,' has an atomic number of 6, meaning it has 6 protons in its nucleus. A neutral carbon atom also has 6 electrons orbiting the nucleus. These electrons are arranged in electron shells: two in the innermost shell and four in the outermost shell, also known as the valence shell. Carbon's electronic configuration is crucial because it dictates its ability to form covalent bonds with other atoms. The valence electrons are the ones involved in chemical bonding, allowing carbon to form stable compounds by sharing electrons with other atoms.
Oxygen (O)
Oxygen, symbolized by 'O,' has an atomic number of 8. A neutral oxygen atom has 8 protons in its nucleus and 8 electrons orbiting the nucleus. The electron configuration of oxygen is two electrons in the innermost shell and six electrons in the valence shell. Like carbon, oxygen's valence electrons determine its bonding behavior. Oxygen's strong electronegativity, meaning its ability to attract electrons, makes it a highly reactive element, readily forming compounds with many other elements.
Formation of Carbon Monoxide (CO)
Carbon monoxide is formed when carbon undergoes incomplete combustion. In complete combustion, carbon reacts with oxygen to form carbon dioxide (CO2). However, when there is a limited supply of oxygen, carbon monoxide is produced instead. The chemical reaction can be represented as: 2C + O2 → 2CO
In this reaction, two carbon atoms react with one oxygen molecule (O2) to produce two molecules of carbon monoxide. Each CO molecule consists of one carbon atom and one oxygen atom held together by a triple bond. This triple bond is exceptionally strong, making the CO molecule relatively stable under normal conditions.
The Significance of the Triple Bond
The triple bond in carbon monoxide is a combination of one sigma (σ) bond and two pi (π) bonds. This arrangement results from the sharing of three pairs of electrons between the carbon and oxygen atoms. The bond order in CO is 3, indicating a strong and short bond. The high bond energy of the triple bond contributes to the molecule's stability, but it also plays a role in its reactivity under specific conditions.
Properties of Carbon Monoxide
The unique atomic structure of carbon monoxide influences its physical and chemical properties. CO is a gas at room temperature, with a boiling point of -191.5 °C and a melting point of -205 °C. Its low molecular weight and nonpolar nature contribute to its gaseous state. Carbon monoxide's most notable chemical property is its ability to bind strongly to hemoglobin in the blood, which is approximately 200-250 times more effectively than oxygen. This strong binding affinity is what makes CO so toxic. When CO binds to hemoglobin, it forms carboxyhemoglobin (COHb), reducing the blood's capacity to carry oxygen. This leads to oxygen deprivation in tissues and organs, resulting in symptoms ranging from headache and dizziness to loss of consciousness and death.
Trends and Latest Developments
Current Emission Levels
Globally, carbon monoxide emissions remain a significant environmental and health concern. Major sources of CO include vehicle exhaust, industrial processes, residential heating, and wildfires. Urban areas with high traffic density often experience elevated CO levels, particularly during peak commuting hours. Efforts to reduce CO emissions involve improving combustion technologies, implementing stricter emission standards for vehicles, and promoting the use of cleaner energy sources.
Impact of Climate Change
Climate change indirectly influences CO emissions. Increased frequency and intensity of wildfires, driven by rising temperatures and drier conditions, lead to higher CO concentrations in the atmosphere. These wildfires release large quantities of CO, contributing to air pollution and posing respiratory health risks to affected populations. Additionally, the changing climate affects atmospheric conditions that influence the dispersion and persistence of CO, further complicating air quality management.
Technological Advancements in Detection
Advancements in sensor technology have led to the development of more accurate and reliable CO detectors. Traditional electrochemical sensors have been refined, and newer technologies, such as infrared (IR) sensors, are becoming more prevalent. These IR sensors offer improved sensitivity and stability, enabling real-time monitoring of CO levels in various environments. Wireless and IoT-enabled CO detectors are also gaining traction, allowing for remote monitoring and alerts, enhancing safety in homes and workplaces.
Regulatory Measures
Governments worldwide are implementing stricter regulations to control CO emissions. These measures include setting emission limits for vehicles and industries, promoting the use of catalytic converters in automobiles, and mandating the installation of CO detectors in residential buildings. Public awareness campaigns play a crucial role in educating people about the dangers of CO poisoning and promoting safe practices, such as proper ventilation and regular maintenance of fuel-burning appliances.
Future Trends
Looking ahead, the integration of artificial intelligence (AI) and machine learning (ML) in CO monitoring and management is expected to grow. AI-powered systems can analyze vast datasets from various sources to predict CO emission hotspots, optimize traffic flow to reduce vehicle emissions, and provide early warnings of potential CO poisoning incidents. Furthermore, research into advanced materials for catalytic converters and the development of more efficient combustion technologies will continue to drive down CO emissions, contributing to cleaner and healthier environments.
Tips and Expert Advice
Installing and Maintaining CO Detectors
One of the most effective ways to protect yourself and your family from CO poisoning is to install CO detectors in your home. Place detectors on each level of your home, especially near sleeping areas and in the vicinity of fuel-burning appliances like furnaces, water heaters, and stoves. Regularly test the detectors to ensure they are functioning correctly, and replace the batteries at least twice a year or as recommended by the manufacturer. Remember to replace the entire unit every five to seven years, as the sensors can degrade over time.
Proper Ventilation Practices
Adequate ventilation is crucial to prevent CO buildup in enclosed spaces. When using fuel-burning appliances, ensure proper ventilation by opening windows or using exhaust fans. Never operate generators or charcoal grills indoors, in garages, or near windows and doors. Regularly inspect and clean chimneys and vents to ensure they are free from obstructions that can impede airflow and cause CO to accumulate.
Regular Appliance Maintenance
Proper maintenance of fuel-burning appliances is essential for safe and efficient operation. Schedule annual inspections and servicing by qualified technicians to ensure that appliances are functioning correctly and safely. Check for any signs of damage, such as cracks or leaks, and promptly repair or replace malfunctioning components. Keep appliances clean and free from debris to prevent incomplete combustion and CO production.
Recognizing the Symptoms of CO Poisoning
Being aware of the symptoms of CO poisoning can help you take timely action and seek medical attention. Common symptoms include headache, dizziness, nausea, vomiting, weakness, confusion, and blurred vision. In severe cases, CO poisoning can lead to loss of consciousness, seizures, and death. If you suspect CO poisoning, immediately move to fresh air and call emergency services. Do not re-enter the premises until it has been properly ventilated and the source of CO has been identified and addressed.
Safe Use of Vehicles and Equipment
Vehicles and equipment powered by internal combustion engines can produce significant amounts of CO. Never run a vehicle in an enclosed space, such as a garage, even with the door open. When using gasoline-powered tools or equipment, operate them outdoors in well-ventilated areas. Ensure that exhaust systems are properly maintained and free from leaks. Be particularly cautious when using such equipment in confined spaces, and always follow manufacturer's instructions and safety guidelines.
FAQ
Q: What is carbon monoxide (CO)? A: Carbon monoxide (CO) is a colorless, odorless, and tasteless gas produced by the incomplete combustion of carbon-containing fuels. It is highly toxic and can be fatal if inhaled in high concentrations.
Q: How many atoms are in one molecule of carbon monoxide? A: There are two atoms in one molecule of carbon monoxide: one carbon atom (C) and one oxygen atom (O).
Q: Why is carbon monoxide dangerous? A: Carbon monoxide is dangerous because it binds to hemoglobin in the blood more effectively than oxygen, forming carboxyhemoglobin (COHb). This reduces the blood's ability to carry oxygen to tissues and organs, leading to oxygen deprivation and potentially death.
Q: What are the common sources of carbon monoxide? A: Common sources of carbon monoxide include vehicle exhaust, malfunctioning furnaces, gas stoves, water heaters, fireplaces, charcoal grills, and generators.
Q: How can I detect carbon monoxide in my home? A: You can detect carbon monoxide by installing CO detectors on each level of your home, especially near sleeping areas and fuel-burning appliances. Test the detectors regularly and replace the batteries as recommended by the manufacturer.
Q: What should I do if my CO detector goes off? A: If your CO detector goes off, immediately move to fresh air, call emergency services, and do not re-enter the premises until it has been properly ventilated and the source of CO has been identified and addressed.
Q: Can carbon monoxide poisoning be treated? A: Yes, carbon monoxide poisoning can be treated with supplemental oxygen. In severe cases, hyperbaric oxygen therapy may be used to help remove CO from the blood more quickly.
Q: Are there long-term health effects of CO poisoning? A: Yes, severe CO poisoning can lead to long-term neurological problems, including memory loss, difficulty concentrating, and personality changes. Prompt treatment can help minimize the risk of these complications.
Conclusion
In summary, a molecule of carbon monoxide (CO) is composed of two atoms: one carbon atom and one oxygen atom. This seemingly simple combination belies the significant impact CO has on both environmental and human health. Understanding its formation, properties, and the risks it poses is crucial for implementing effective safety measures and reducing its emissions.
By installing and maintaining CO detectors, practicing proper ventilation, and ensuring regular appliance maintenance, we can mitigate the dangers of CO poisoning. Remember, awareness and proactive measures are key to protecting ourselves and our communities from the "silent killer." Take action today to ensure your home and workplace are safe from the hazards of carbon monoxide. Share this information with your friends and family to help spread awareness and promote safety.
Latest Posts
Latest Posts
-
How Many Yards In 100 M
Dec 06, 2025
-
How Do You Spell Color Grey
Dec 06, 2025
-
What Year Did Exodus Take Place
Dec 06, 2025
-
How Many City Are In Tennessee
Dec 06, 2025
-
How Does An Electric Current Flow
Dec 06, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In Co . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.